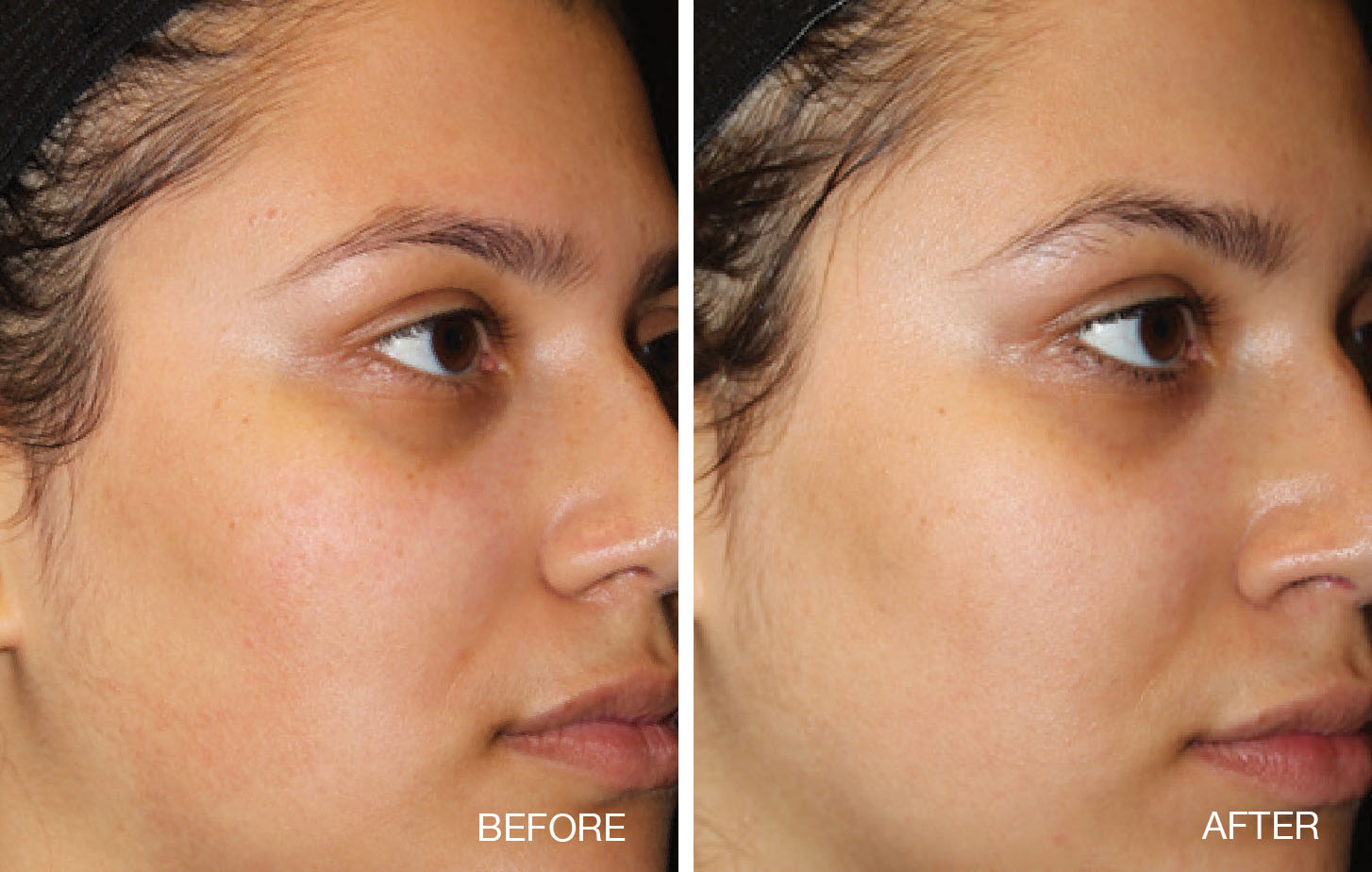
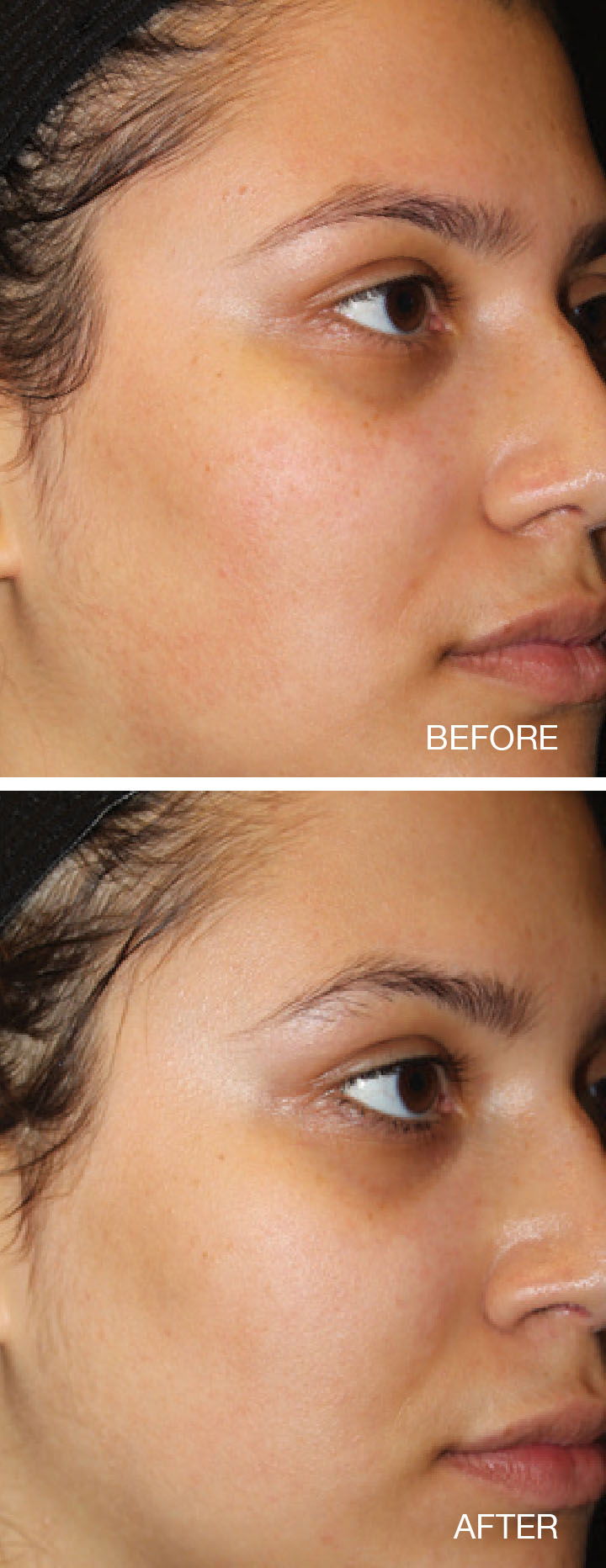
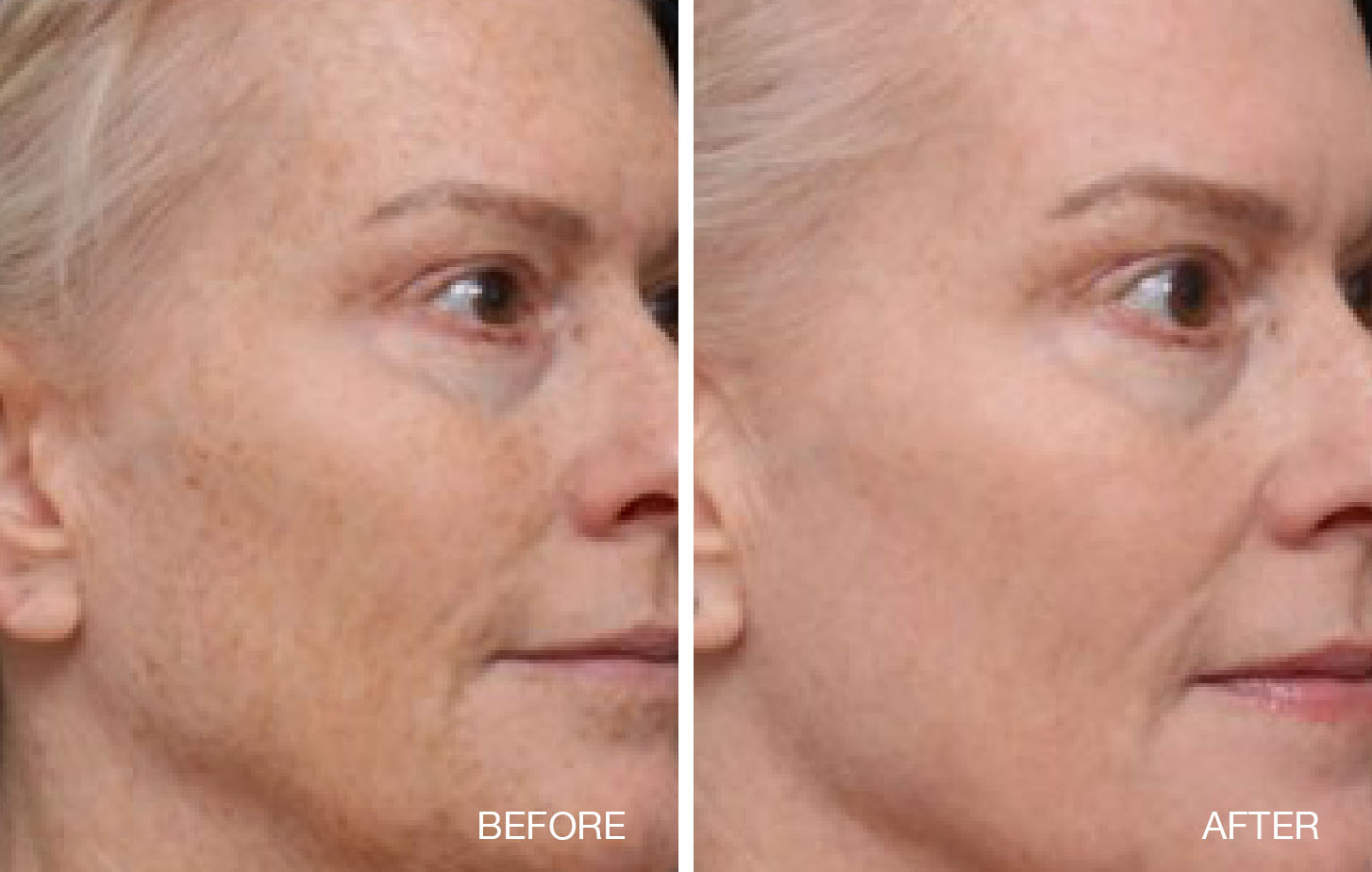
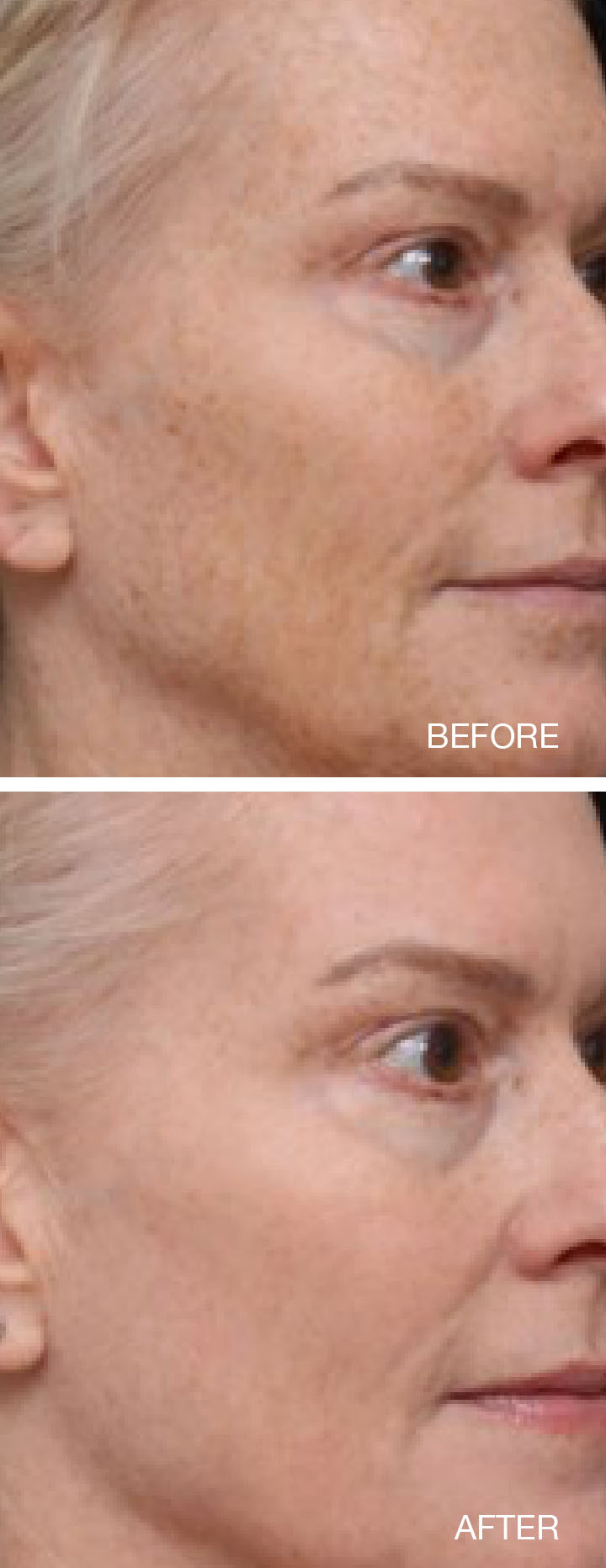
Thermage® System Indications and Important Safety Information
INDICATIONS
- The radiofrequency energy only delivery components of the Thermage® FLX
System and accessories are indicated for use in:
- Dermatologic and general surgical procedures for electrocoagulation and hemostasis;
- Non-invasive treatment of periorbital wrinkles and rhytids including upper and lower
eyelids
- Non-invasive treatment of wrinkles and rhytids
- The simultaneous application of radiofrequency energy and skin vibration by the
Thermage® FLX System and accessories are indicated for use in:
- Dermatologic and general surgical procedures for electrocoagulation and hemostasis;
- Non-invasive treatment of periorbital wrinkles and rhytids
- Non-invasive treatment of wrinkles and rhytids
- Temporary improvement in the appearance of cellulite
- Relief of minor muscle aches and pain
- Relief of muscle spasms
- Temporary improvement of local circulation (blood circulation)
IMPORTANT SAFETY INFORMATION
Contraindications: The Thermage® FLX System is contraindicated for use in
patients with an implantable pacemaker, an automatic implantable cardioverter / defibrillator
(AICD), or any other implantable electrical device, as these devices may be adversely affected by
radiofrequency (RF) field or current.
Precautions:
- Solta Medical has not studied the use of the Thermage® system:
- Over skin fillers (lips, cheeks, facial wrinkles and skin folds)
- In people who are pregnant and/or breast feeding, are diabetic, have an auto-immune
disease
such as lupus, have cold sores, have herpes simplex, or have epilepsy
- In people with permanent makeup and/or tattoos
- In children
Warnings:
- Treatment of the upper or lower eyelids, or within the orbital rim, requires the use of PLASTIC
eye shields, DO NOT use metal shields.
- Do not use vibration when treating the face around the eye areas while plastic eye shields are
in use. Vibration may cause displacement of the ocular shields resulting in increased risk of
corneal abrasions or ocular damage.
Possible Adverse Patient Reactions:
Reports of adverse patient reactions have included:
- During treatment: mild-to-moderate pain
- After treatment:
- Edema
- Erythema or blanching
- Urticaria
- Bruising
- Blisters, burns, scabbing or scarring
- Surface irregularities
- Altered sensation
- Lumps or nodules
- Pigment change
- Rare cases of herpes simplex virus reactivation in the perioral and genital skin areas
ATTENTION: THIS IS NOT ALL YOU NEED TO KNOW. Improper use of the
Thermage® FLX System may cause personal injury, injury to the patient, or damage to the
system. See the User’s Manual and other labeling for detailed directions, proper use, and full risk
and safety information.
For additional information see www.thermage.com/hcp.
CAUTION: Federal (USA) law restricts this device to the sale by or on the order of a
physician.
The Thermage system is Rx Only. See the Operator Manual for detailed directions, proper use,
and full risk and safety information. For additional product information, see www.thermage.com/hcp.
Fraxel FTX™ Laser System Indications and Important Safety Information
INDICATION
Fraxel FTX™ Laser System is indicated for:
- Fraxel® 1550nm wavelength is indicated for dermatological procedures
requiring the coagulation of soft tissue; skin resurfacing procedures; treatment of dyschromia
and cutaneous lesions, such as, but not limited to lentigos, solar lentigos, actinic keratosis,
and melasma; and treatment of periorbital wrinkles, acne scars, and surgical scars.
- Fraxel® 1927nm wavelength is indicated for dermatological procedures
requiring the coagulation of soft tissue; treatment of actinic keratosis; treatment of pigmented
lesions, specifically age spots, sun spots, and ephiledes.
IMPORTANT SAFETY INFORMATION
Contraindications
- Do not use on any patient who is ineligible for general surgery.
- Pre-screening should include, but not be limited to:
- Predisposition to keloid formation or excessive scarring;
- Changes following surgery;
- Skin indentations and textural changes following surgery;
- Systemic steroids (e.g. prednisone, dexamethasone), which should be
rigorously avoided prior to and throughout the course of treatment; and
- Patients undergoing isotretinoin acne treatment or with drugs in a similar class.
- Medical judgement should be used when treating patients with a predisposition to post
inflammatory hyperpigmentation (PIH).
Warnings and Cautions
- Protective eyewear or goggles should be worn by any patient, operator and assistant.
- Medical judgement should be used when treating patients with certain medical conditions.
- Improper use of Fraxel® may cause personal injury or damage to the system.
Adverse Events
- The following may be associated with Fraxel® laser treatment: Blistering and burns;
Temporary or permanent skin discoloration; Eye injury; Infection; Keloid formation; Prolonged
redness; Scarring; Delayed wound healing / skin textural changes and Temporary bruising.
The Fraxel FTX™ Laser System is Rx Only. See the Operator Manual for detailed
directions, proper use, and full risk and safety information. For additional product information
se www.fraxel.com/hcp.
Clear + Brilliant® Laser System Indications and Important Safety
Information
INDICATION
Clear + Brilliant® laser system (1440nm and 1927nm handpieces) is indicated for
dermatological procedures requiring the coagulation of soft tissue and general skin resurfacing.
IMPORTANT SAFETY INFORMATION
- The following contraindications are routine for many laser treatments and may also be associated
with non-ablative Clear + Brilliant® laser system treatments. Pre-screening should
include, but not be limited to:
- Diagnosis / possibility of actinic keratosis, melasma, rosacea, or other significant
skin conditions (e.g., skin cancer, active infections, cold sores, open wounds, rashes,
burns, inflammation, eczema, psoriasis);
- Predisposition to keloid formation or excessive scarring;
- Diagnosis of a condition that may compromise the immune system, such as HIV, lupus,
scleroderma, or systemic infections;
- Known sensitivity to light or if photosensitizing agents or medications are being taken;
- Systemic steroids (e.g., prednisone, dexamethasone), which should be rigorously avoided
prior to and throughout the course of treatment;
- Use of retinoids, which should be avoided for at least 2 weeks prior to and during
treatment;
- Patients undergoing isotretinoin acne treatment or with drugs in a similar class;
- Whether skin is still recovering from a cosmetic procedure, such as a chemical or
mechanical peel, or laser resurfacing; and
- Whether botulinum toxin injections, or dermal fillers (such as collagen) have occurred
within the past 2 weeks.
- Expected responses to treatment include, Erythema, Edema, Itching or Dryness, Increased
Skin Sensitivity, Pain or Discomfort, and Petechiae.
Improper use of Clear + Brilliant® may cause personal injury or damage to the
system. See the Operator Manual for detailed directions, proper use, and full risk and safety
information. For additional product information visit www.clearandbrilliant.com/hcp.
CAUTION: Federal (USA) law restricts this device to the sale by or on the order of a
physician.